Consultation on the Global Definition Consensus Conference
The committee obtained comments from members of a convenience sample of 21 global critical care societies. No requests for formal societal endorsements were made, and the comments do not reflect the official views of individual professional societies. Comments were reviewed and considered by the committee.
Additional comments are welcome and can be posted here.
Comments
- Absolutely agree that these changes are timely and reflective of the evidence. Additionally, expansion of the definition for resource poor settings is long overdue.
- I have mixed feeling about broadening diagnosis in an already very heterogeneous ‘disorder’ I would prefer identifying characteristics that would focus on specific ‘types’ leading to more personalized interventions.
- Racial bias in S/F must be highlighted.
- In general, the use of “treatment” criteria as part of definition adds subjectively and likely over-broadens the definition unless linked specifically to other criteria (high flow nasal oxygen to achieve SpO2 or PaO2 goal).
- These are situations where PaO2/FiO2 or SpO2/FiO2 ratio cutoffs vary with FiO2, sometimes enough to meet/not meet criteria for definition.
- The rate of 30L HFNO – is this for the oxygen component only? Thus, 30L HFNC at 100% FiO2 but not 40L HFNC at 60% FiO2 would satisfy the oxygen requirement for diagnosis? This needs to be firmly established by any new guideline document.
- The implementation of S/F cutoffs would be reasonable but given the recent data regarding the unreliability of these measurements in patients with darker skin colors would give me some pause about their generalizability.
- Clear rules will be required for use of ultrasound to demonstrate bilateral infiltrates – for example, >3 B lines per rub space of at least 18 cm depth, in at least three different rib spaces over each hemithorax. I’m sure these are already being considered but they would be important concerns I would like to see addressed within the policy document.
- Flow rate for HFNC needs to be higher.
- Would need to standardize way to impute SpO2/FiO2 in ventilated and non-ventilated patients that is better than existing methods and accounts for hidden hypoxemia.
- Consider eliminating rapidly resolving ARDS i.e. aspiration pneumonitis that gets better
- The ultrasound literature in general suggests quite a bit of variability in assessing for pulmonary edema, and I worry about misclassification of ARDS.
Responses
- We agree with the priority of improving phenotyping and individualized interventions in ARDS. This is an area of ongoing research.
- We have highlighted the possible drawbacks of SpO2/FiO2, including potential racial bias, in the comments section of our manuscript.
- It is true that oxygenation may vary by the device and support settings; this is also a limitation of the Berlin definition, but to maintain face validity we felt that a minimum level of support in areas where this is feasible (excluding resource-limited areas) was necessary. We acknowledge that there are limitations to this approach.
- The rate of 30 L/min is for the flow rate. The FiO2 component is addressed by the PaO2/FiO2 or SpO2/FiO2 criterion, so either of the proposed scenarios might qualify as ARDS depending upon the PaO2/FiO2 or SpO2/FiO2 ratio.
- We have emphasized that ultrasound operators must be trained in the use and interpretation of thoracic ultrasound; however, since there are no strict criteria for what constitutes infiltrates on chest x-ray or computed tomography, we have also not provided strict criteria for ultrasound. This may be an area of future research (i.e., scoring systems such as the RALE score); however, there is not currently evidence to support specific criteria for identifying infiltrates for any imaging modality, since chest x-ray also has limitations with inter-rater reliability.
- Please see comments above regarding the flow rate for HFNO.
- We have chosen the Rice equation for imputation of SpO2/FiO2. Because there is not currently a temporal component to the definition of ARDS, we have not added an exclusion for rapidly resolving ARDS.
Comments
- Excellent expanded definition of ARDS and these modifications will help broaden the studies in ARDS to under-resourced settings.
- The use of SpO2 as a surrogate. May leads to inaccuracies due to the documented inaccuracies in people of color.
- The evidence of need for bilateral infiltrates (vs any infiltrate) is poor.
- Resource poor nations do not need separate criteria. A standard need to be set. If other definitions created, how does that affect the data and future research. Don’t do it.
- I am hesitant to include the S/F ratio without knowing that you actually can correct for inherent racial biases. I think it is dangerous to include the pulse oximeter into the definition until that device has been shown to be equitable.
Responses
- The Committee recommendations for further research include studies of the basis for discrepancies between SpO2 and PaO2. Currently, however, the requirement for PaO2 also exacerbates inequities by removing the possibility of diagnosing ARDS in settings where this modality is not available. On balance, we believe that the advantages of early recognition of ARDS, including in settings where PaO2/FiO2 is not readily available, outweigh the potential disadvantages of using SpO2/FiO2, but more research is needed to improve accuracy in patients with different degrees of skin pigmentation.
- The Committee acknowledges the potential value of a standard definition that can be applied worldwide, but as long as resources for diagnosis and clinical care vary markedly around the world, more flexibility is needed in order to reduce the chance of missing patients with the same pathophysiology who are cared for in resource limited settings. The Kigali modification of the definition of ARDS has been of great value and is recognized in the expanded definition of ARDS.
Comments
- We believe that these changes bring positive options for the management of patients with ARDS.
Comments
- Suggest consideration of at least 50L/min with mouth closed to be more in line with NIV.
- Since SpO2 errors are mentioned, for completeness, mention of errors of blood gas measurements may be considered. Suggest including pseudohypoxemia in hyperleukocytosis where ABG blood gas may give falsely low reading compared to actual in vivo PaO2.
Responses
- Experience during the COVID-19 pandemic showed that there is not a consistent oxygen flow rate that defines HFNO. The committee recognized that 50L/min was used in the original study that supported use of HFNO, but recommends using a flow rate of 30 L/min in order to include more patients with the pathophysiology of acute lung injury, including in settings where HFNO cannot be delivered at flow rates above 40 L/min. Additionally, even 30 L/min provides a degree of PEEP.
Comments
- We do agree with using HFNO definition and S/F ratio as alternative to P/F and support modification definition in resource limited settings.
- Do not agree with allowing ultrasound to diagnose bilateral infiltrates due to excessively operator dependence.
Responses
- Consensus Conference recommendations specify that ultrasound should be used only where the operator(s) have appropriate training. Additionally, inter-rater reliability even with chest radiograph is poor.
Comments
- Endorse the findings from our own 50-participant study, which concludes that SpO2/FiO2 (SF ratio) can be used for diagnosis and classification of ARDS as a substitution of PaO2/FiO2 (PF ratio).
Comments
- The efforts to simplify and globally expand the ARDS definition seem extremely helpful to research and clinicians worldwide.
- Regarding the definition for resource variable settings: if an end-expiratory pressure or a minimum flow rate is not required, we think that the SpO2/FiO2 should be less than 315, perhaps 235.
Responses
- The Consensus Conference recommended that the ARDS severity categories defined in the Berlin Definition be retained. The SpO2/FiO2 = 315 is used in the Kigali modification of the Berlin definition and is consistent with the upper limit of the “mild ARDS” category (PaO2/FiO2 < 300 mmHg) in the Berlin definition, so the SpO2/FiO2 = 315 is recommended as the oxygenation cutoff when arterial blood gases are not available.
Comments
- We do not believe that the expansion of ARDS diagnostic criteria under these conditions will contribute to patient assessment or classification.
- We believe that HFNC will be overused, and that SpO2/FiO2 will be better in mild cases but not in moderate or severe cases.
Responses
- The Committee agrees that HFNO is now widely used in resource intense as well as resource limited settings; however, the requirements for hypoxemia in conjunction with HFNO will still capture patients who have a severe oxygenation defect. The SpO2/FiO2 ratio performs well in the moderate and severe categories of ARDS.
Comments
- With the deepening understanding of ARDS, especially the changes in critical care practice since the COVID-19 pandemic, we believe it is necessary to expand the definition of ARDS. The expanded criteria are of great value.
Comments
- The integration of High Flow Nasal Oxygen, ultrasound and SpO2/FiO2 ratio as an alternative is reasonable and correct.
- I would not include the modified definition for ARDS in resource variable settings; in my opinion, it dilutes the definition and further reduces acceptance.
- However, this now makes the definition more complex. The more complex the definition becomes, the more difficult this may become.
- Would emphasize that arterial blood gas is the first choice if available, and the SpO2/FiO2 ratio is the second choice. SpO2/FiO2 ratio may not be optimal for clinical trials.
- PaO2/FiO2 may differ between HFNO and invasive mechanical ventilation due to PEEP which might be relevant in clinical trials.
Responses
- The Kigali modification has been very useful for identifying ARDS in resource limited areas, and the Committee emphasizes that the diagnosis of ARDS should not be limited by care setting (i.e., ARDS exists in countries where blood gases and mechanical ventilation are not available). We agree that in the clinical trial setting, investigators may choose to use PaO2/FiO2 as the preferred hypoxemia criterion; however, SpO2/FiO2 has been successfully utilized in ARDS research. The Committee acknowledges that PEEP influences the PaO2/FiO2 ratio; this is also true with different PEEP settings for patients who are mechanically ventilated, and measurements will always be somewhat variable depending upon respiratory support. Again, investigators may choose to require specific devices or settings as they deem appropriate
Comments
- The proposed expanded definition was comprehensive.
- The line ‘ARDS is an acute diffuse, inflammatory lung injury precipitated by a predisposing risk factor such as pneumonia, non-pulmonary infection, trauma, transfusion, burn, aspiration, or shock’ does not include other inflammatory states like burns, pancreatitis, Post cardiac surgery pump etc. Suggestion: ARDS is an acute diffuse, inflammatory lung injury precipitated by a predisposing risk factor such as pneumonia, non-pulmonary infection, and non-infectious inflammatory states including but not limited to trauma, transfusion, burn, aspiration, pancreatitis and shock’
- Suggestion: The resulting injury leads to disruption of alveolar-vascular interface causing increased pulmonary vascular and epithelial permeability, lung edema, and gravity-dependent atelectasis, all of which contribute to loss of aerated lung tissue.
- Suggestion: The clinical presentation is influenced by medical management (position, ventilator settings, sedation, paralysis, and fluid balance).
Responses
- In point #1, the list of clinical factors was not meant to be exhaustive, so we have added “or other factors” to the end of the first sentence in the conceptual model section to address the reviewer’s comment. Points two and three are very close to the existing text, so further edits have not been made.
Comments
- Delighted to endorse this new expanded definition of ARDS.
- No modifications to proposal.
Comments
- We can confirm the endorsement of the proposal by the Kuwait Anesthesia and Critical Care Society.
- No modifications to proposal.
Comments
- Generally agree to most points.
- COVID-19 timing should be extended to be longer than a week.
Responses
- The Committee agrees that there should not be a firm time limit for the definition of ARDS. The Committee recommendation is that the onset should be within approximately one week.
Comments
- We think it will lead to a reworking of the treatment guidelines and introduce some biases into the treatment, but indeed we validate this expanded definition. Well written and we agree with the expanded definition that we find valuable and adapted to low and middle resources settings.
- No minor comments
Comments
- Approve new expanded definition of ARDS.
Comments
- I think that these modifications will help broaden the studies in ARDS to under-resourced settings.
- There needs to be clarification that the patient could not be adequately oxygenated without the use of HFNC. Just being on HFNC isn’t enough.
- The benefit of making a diagnosis is that it is specific so that treatment can be tailored to that diagnosis. ARDS is a condition of lower lung compliant. Not diffuse ground glass infiltrates and hypoxia. I would be in favor of a definition that centered on lung compliance and not saturation (too many confounders). This compliance definition would take into account body habitus, presence of effusions, etc. The key thing we are trying to prevent is ventilator induced lung injury and superoxia induced lung injury and atelectasis. I feel these changes ignore lung compliance and open the diagnosis of ARDS up too broadly to make clinical impact irrelevant and clinical research outcomes too dilute.
Responses
- The Committee acknowledges that lung compliance I reduced in many patients, but data from patients with COVID has shown that lung compliance can eb quite variable. In addition, measuring lung compliance is not possible in all settings. Therefore, measurement of lung compliance was not included in the expanded definition of ARDS.
Comments
- This is a nice effort to enable easier and more inclusive identification of ARDS in practice.
- The category for non-intubated ARDS may not be needed since the modified definition for resource-variable settings already allows for ARDS definition w/o the need for mechanical ventilation. In addition, the PEEP effect of HFNO is minimal and variable anyway, especially if patients breathe through their mouths.
- The threshold values for S/F ratio between mild and moderate ARDS overlap at 235.
- It may not be possible to exclude masses on lung ultrasound.
Responses
- The category for non-intubated ARDS is included for clarity since the modified definition for resource-variable settings has no device requirement, but in other settings HFNO or NIV at a minimum setting is required. The comment about expiratory pressure in Table 1 applies to patients on NIV/CPAP.
Comments
- Support all element of the expanded definition.
Polling data from international critical care societies
Members of some societies were polled by either Google Poll or RedCap Survey to obtain responses to the 4 questions below.
- Do you agree with expanding the Berlin definition of ARDS to include High-Flow Nasal Oxygen with a minimum flow rate of 30L/minute?
Yes 89% (169)
No 11% (21)
- Do you agree with adding the SpO2/FiO2 ratio as an alternative to the PaO2/FiO2 to the oxygenation criteria?
Yes 82% (154)
No 18% (34)
- Do you agree with allowing the use of ultrasound to diagnose bilateral infiltrates?
Yes 67% (128)
No 33% (62)
- Do you agree with the modified definition of ARDS in resource variable settings (the Kigali modification as published in AJRCCM 2016), specifically to diagnose ARDS in resource limited settings only?
Yes 81% (153)
No 20% (37)
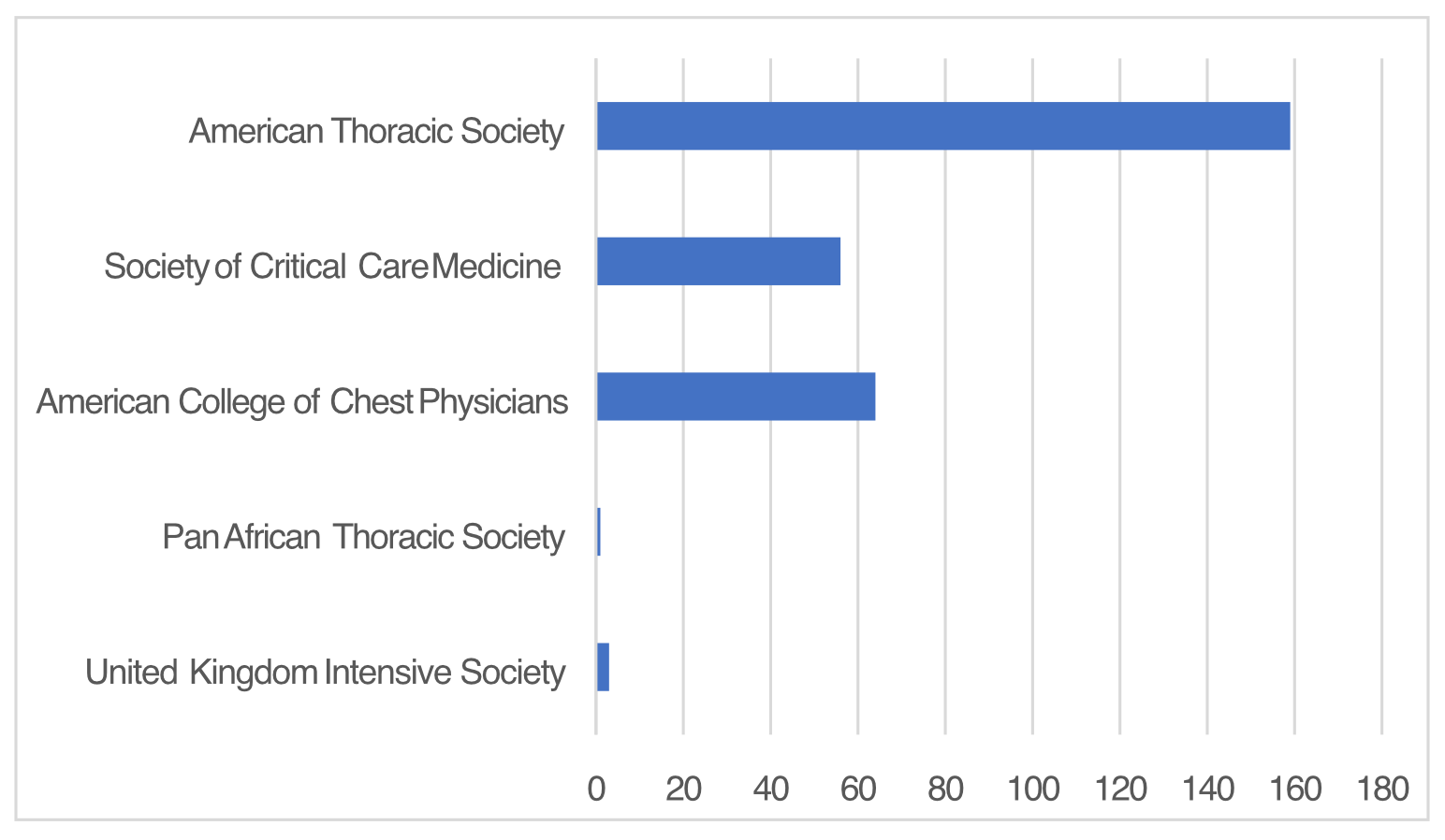
The number of individual responses from each soceity participating in polling.
Am. J. Respir. Crit. Care Med. 2023. doi: 10.1164/rccm.202303-0558WS. Online ahead of print.
Comments from members of international critical care societies